Introduction:
Bleeding disorder of unknown cause (BDUC) is a diagnosis of exclusion and patients share a similar phenotype with established mild bleeding disorders (MBDs) such as platelet function defects (PFD), mild von Willebrand disease (VWD), or mild coagulation factor deficiencies (CFD).
The integration of global assays, such as the Platelet Function Analyzer (PFA)-100, as screening tools for MBDs lacks universal endorsement and is currently not recommended in clinical routine. While the PFA-100 has demonstrated noteworthy sensitivity in the detection of VWD, data regarding its clinical utility in PFD are conflicting and data on PFA-100 in BDUC patients are scarce.
Aims:
To evaluate the diagnostic utility of PFA-100 in BDUC compared to other MBDs, and to investigate the influence of various parameters on PFA-100 results.
Methods:
This study included 821 patients enrolled in the Vienna Bleeding Biobank (VIBB), a single-center cohort study on individuals with non-trivial bleeding tendencies who were referred to our tertiary center until December 2022 (EC 604/2009). Patients under anticoagulation or antiplatelet treatment were excluded. PFA-100® assessments (Dade Behring Inc., Newark, Delaware, USA) were conducted on citric acid anticoagulated whole blood samples obtained at study inclusion using two different cartridges: Dade® PFA-100 Collagen/Epinephrine (EPI) and Dade® PFA-100 Collagen/adenosindiphosphat (ADP; Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany).
Results:
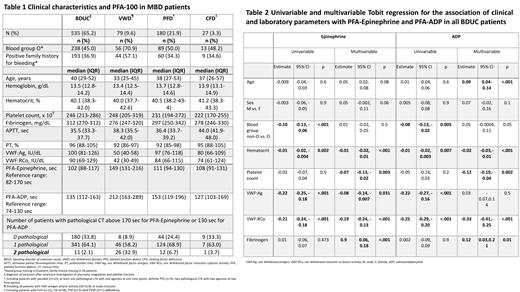
PFA-100 data of 535 BDUC patients (65%) were compared to those of 79 patients (10%) with VWD, 180 (22%) with PFD, and 27 (3%) with CFD. Clinical characteristics are shown in Table 1.
In patients with BDUC, the median (interquartile range) closure time (CT) measured by the PFA-100-EPI assay was 102 seconds (88-117), and 135 seconds (112-163; Table 1) with the PFA-100-ADP assay. The CTs were shorter in BDUC compared to VWD (PFA-100-EPI: p<.001, PFA-100-ADP: <p.001) and PFD (PFA-100-EPI: p<.001, PFA-100-ADP: <p.001), while no significant difference was observed compared to CFD (PFA-100-EPI: p=0.2; PFA-100-ADP: p=0.7).
Based on in-house reference ranges, CT was normal in both PFA-100 assays in 180 (34%) of all BDUC patients, while 341 patients (64%) had at least one abnormal CT and 11 patients (2%) had abnormal CTs in both PFA-100 assays. A similar distribution was observed in patients with CFD (no abnormal CT: 33%, 1 abnormal CT: 63%, 2 abnormal CTs: 4%). Statistical analysis revealed a significant difference in the number of pathological CTs between BDUC and VWD (no abnormal CT: 9%, 1 abnormal CT: 58%, 2 abnormal CTs: 33%; p<.001), as well as between BDUC and PFD (no abnormal CT: 24%, 1 abnormal CT: 69%, 2 abnormal CTs: 7%; p=0.003).
In respect to bleeding severity, in BDUC patients no correlation was found between CTs in the PFA-100-EPI and PFA-100-ADP assays with the Vicenza bleeding score (PFA-100-EPI Spearman rho (r)=0.06, p=0.06; PFA-100-ADP r=0.02, p=0.6), ISTH-BAT (PFA-100-EPI r= 0.06, p=0.3; PFA-100-ADP r= 0.10, p=0.06), or the number of bleeding manifestations (PFA-100-EPI r=0.01, p=0.81; PFA-100-ADP r=-01, p=0.8). The number of pathological PFA-100 was not associated with specific bleeding symptoms assessed by the Vicenza score in BDUC (data not shown).
Using a Tobit regression model, we analyzed the association of clinical and laboratory parameters with prolonged CTs in both PFA-100 assays in BDUC, as shown in Table 2. In multivariable linear regression, reduced hematocrit, lower VWF:Ag/VWF:RCo levels, reduced platelet count and higher fibrinogen levels were associated with longer CTs. Increasing age was additionally associated with a longer CT in PFA-100-ADP.
Conclusion:
In a minority of BDUC patients CT was prolonged in both PFA-100 (EPI and ADP) assays. PFA-100 was not associated with the bleeding severity or specific symptoms in BDUC. In BDUC patients, lower hematocrit, lower platelet count, lower VWF levels and interestingly higher fibrinogen levels were associated with a prolonged CT. Our descriptive results do not suggest the integration of PFA-100 as screening tool for BDUC patients.
Disclosures
Mehic:CSL Behring: Honoraria. Ay:Bayer, CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi and Takeda: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal